Want to learn more about what a plasticizer is? Check out this great informational video:
Bio-Based Feedstock Movement Gaining Momentum
Europe has found a future in agricultural production in the north-west corner of the continent. Researchers have found that the cost to produce sugar in that part of the continent would be among the lowest in the world. The estimate by Deloitte is that Europe could produce nearly 30% more agricultural products than they already are, rivaling the sugar cane production in Brazil as well as corn production in the Midwest United States. Bio-based industries currently make up about 1% of the European Economy, where as the investors want to look for a way to bolster that to around 10%. This is now possible, and with this new development in the prosperity of the European land this now means that 95% of all products today can be bio-based.
For more information see ICIS.com
Scientists Create New Superchilled Compound
The notion of absolute zero has been around for quite some time. Lowering the temperatures of a molecule as close to zero as possible could reveal some wacky physics that could reveal some properties on exotic states of matter. Now, a team at MIT led by physicist Martin Zwierlein, was able to cool down sodium potassium gas using a process involving lasers to dissipate the energy of individual gas molecules. They succeeded in chilling the molecules as low as 500 nanokelvins, which equates to -459.67˚F. This is interesting because sodium and potassium don’t usually form compounds since both are positively charged and would typically repel each other. Although they achieved the formation of this compound, it wasn’t as stable as an everyday chemical. It only lasted 2.5 seconds before it broke apart, but when dealing with such extreme conditions that is a very long time. This may not seem like a great advancement, but it could shed some light on mysteries such as dark energy, the mysterious substance that is apparently pulling our universe apart.
For more information see Business Insider.
Castor Crops May have a Future in the U.S.
Due to the federal government ceasing their price supports in 1972, the Castor crop has not been grown in the U.S. since then. Because of this, the U.S. had no choice but to turn to producers in China, India, and Brazil to supply its needs. But now, according to a new University of Florida study, the Castor plant can be grown in Florida using proper management techniques. Since the Castor industry is quite large already, this development indicates an immense economic growth potential.
For more information visit ScienceDirect.com.
Researchers Develop Catalyst to Remove Benzene From Gasoline
Benzene, which is an aromatic hydrocarbon and a natural constituent of crude oil, has been recognized by the Environmental Protection Agency as a known contributor to cancer. According to the U.S. Energy Information Administration, an estimated 137 billion gallons of gasoline were consumed in the United States last year. This, unfortunately, makes benzene a substantial environmental and health problem. But recently, strides have been made to remove benzene from gasoline. Northwestern University scientists have developed a catalyst that has effectively removed benzene from the other aromatic compounds in gasoline, making it cleaner and more efficient. The catalyst is described as an organometallic molecule. While normally, the molecule is composed of expensive platinum, this catalyst is made from an affordable simple metal that is absorbed onto a particular oxide support. Since the process of removing benzene would be inexpensive, this could keep the cost of gasoline down in the future.
For More Information see Phys.org.
New Guidelines For GHS Safety Data Sheets (SDSs) Go Into Effect
The deadline for all chemical manufacturers, importers, and distributors to be in compliance with the new guidelines for Safety Data Sheets (SDSs) has passed. Beginning December 1st a new requirement for chemical labeling will also go into effect. OSHA first announced the changes in 2012 when they revised the Hazard Communication Standard (HCS, HazCom) to align with the Globally Harmonized System (GHS). More recently, by the request of the National Association of Chemical Distributors (NACD), OSHA released written guidelines clarifying key points of the changes such as whether or not distributors can continue to ship older stock products that comply with the old labeling standard. Please see the full communication at the NACD website below.
NACD Welcomes OSHA Guidance on Hazard Communication Standard.
Polymer Wrapped Carbon Nanotubes
Carbon nanotubes (CNTs) were first discovered in the early 1990s. They are 100 times stronger than steel and one-sixth the weight, have several times the electrical and thermal conductivity of copper and lack most of the environmental or physical degradation issues related to most metals. The drawback is that CNTs have a tendency to aggregate into clumps, where their properties are best utilized when dispersed. Adding to the difficulty is that CNTs are insoluble in many liquids, making even dispersion difficult. A new method has been developed at Japan’s Kyushu University and reviewed by Dr. Tsuyohiko Fujigaya and Dr. Naotoshi Nakashima that “exfoliates” aggregated clumps of CNTs and disperses them in solvents. The technique is called non-covalent polymer wrapping and it works by wrapping the CNTs in a polymer using a non-electron sharing bond. Non-covalent bonding was chosen because covalent bonding, the sharing of electrons within the bond, can change the intrinsic properties of the carbon nanotubes, where non-covalent has minimal effects in most cases. A wide variety of polymers were found to be able to disperse the CNTs and many have been able to add new functions to the tubes. This research has implications in biomedicine and to improve photovoltaic functionality, as well as other fields.
For more information see Phys.org.
ChemCeed Teaches Students About Polymers
 ChemCeed Intern, Marcos Waksman, teaches students about polymer chemistry at this year’s Career Venture held yesterday in Eau Claire. The ChemCeed table was visited by local middle and high school students, and the ChemCeed staff showed them how to make bouncy balls to demonstrate polymer chemistry.
ChemCeed Intern, Marcos Waksman, teaches students about polymer chemistry at this year’s Career Venture held yesterday in Eau Claire. The ChemCeed table was visited by local middle and high school students, and the ChemCeed staff showed them how to make bouncy balls to demonstrate polymer chemistry.
Advancement In Non-Stick Surfaces
LiquiGlide, a company started by Kripa K. Varanasi, a professor of mechanical engineering at M.I.T., and J. David Smith, a graduate student of Dr. Varanasi’s, has developed a non-stick coating which traps a lubricant on a rough surface. Similar research has been done using superhydrophobic surfaces, where air is trapped on the rough surfaces, allowing liquids to flow past. When liquids flow, the layer in contact with a surface sticks, creating a more viscous fluid. This can be seen in pipes, where the liquid on the edge flows slower than the liquid in the center. The technology can be applied to highly viscous materials called Bingham plastics, materials which require a force to flow. Using trapped air has been effective but the microscopic surfaces in which they’re trapped can become damaged, allowing liquid to displace the air and creating more viscosity. Air may also dissolve into the liquid when submerged for a long period of time. LiquiGlide develops a lubricant specifically for the liquid, using a theory to predict interactions among the surface, the lubricant and air. The lubricant binds more strongly to the textured surface than to the liquid, allowing the liquid to slide on a layer of lubricant. The textured surface keeps the lubricant in place. Dr. Varanasi originally began his research into industrial challenges like preventing ice from forming on airplane wings and allowing more efficient pumping of crude oil and other viscous liquids. But recently LiquiGlide announced that Elmer’s Products Inc. had signed an exclusive licensing agreement for the use of their coatings in glue containers, and has licensed its technology to an Australian company to be used on the inside surface of paint can lids.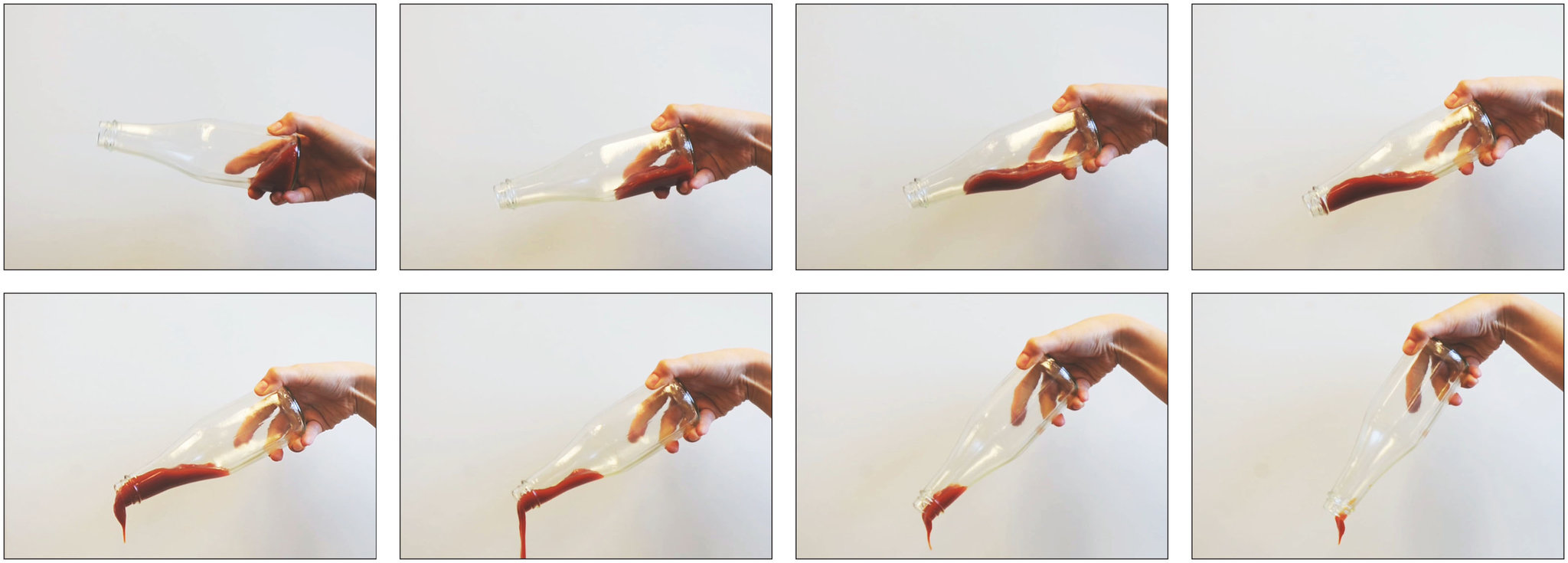
Key-Protein Identified In Dandelion Rubber Production
In a joint effort, researchers at Münster University, the Münster branch of the Fraunhofer Institute for Molecular Biology and Applied Technology IME, the Technishe Universität München (TUM) and TRM Ltd. (York, UK) have found what they believe to be the key-proteins involved in the production of rubber in dandelions. They were able to demonstrate using the Russian dandelion, Taraxacum kok-saghyz, as an example of a special protein, a so-called rubber transferase activator. If this protein is lacking, the plant does not produce rubber. With their findings they believe the protein is necessary for the formation of the rubber-producing protein complex. In a second study, with input from IME and Münster University, another protein was found which plays a key role in the formation of the long polyisoprene chains, the polymers which give rubber its elasticity and resilience. This research is hoped to be used to biotechnologically produce natural rubber and advance research in the role of rubber in plants.
For more information see Phys.org.
